Accurate Performance Verification with qPCR
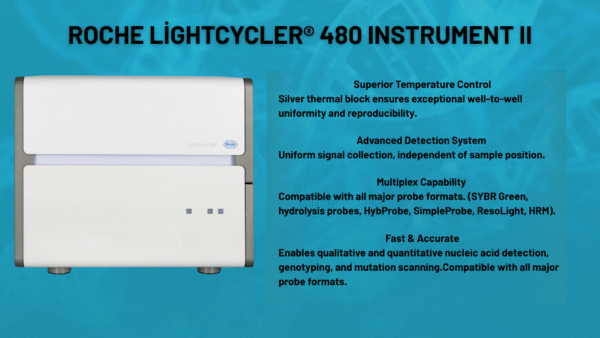
Our qPCR service is designed not for diagnostic purposes, but to verify whether your products function as intended, identify the source of problems, and confirm assay reliability. Using the Roche LightCycler® 480 II system, we deliver highly sensitive and reproducible results.
If needed, we can perform DNA isolation from your biological samples prior to qPCR analysis, ensuring optimal quality, purity, and highly reliable results.
Key Features
| High-sensitivity qPCR instruments for accurate and reproducible results |
| Experienced technical team and optimization support |
| Minimal error margin and reliable data analysis |
| Quality control-verified results |
| Optional Isolation Step |
| Flexible Sample Acceptance |
When to Use Our qPCR Service?
- Determining whether a primer/probe set is functioning correctly
- Identifying the specific product causing a problem in your workflow
- Detecting issues in multi-set panels and pinpointing the defective set
- Verifying performance before large-scale testing or production
Minimum Technical Requirements
To perform the qPCR test, our customers must provide the required samples and reagents prepared in accordance with the purpose and method of the test. This is essential to ensure the accuracy and reliability of the results:
Appropriate reaction mixture (Master Mix)
Primer and probe sets (compatible format)
Positive control samples (DNA, cDNA or Synthetic Positive Control)
Additional materials should also be provided depending on the assay design.
Provided Data
Upon completion, you will receive:
- A detailed report including amplification curves, Cp values, and interpretation notes
- Raw qPCR data for your records
- Recommendations for troubleshooting or optimization if issues are detected