Ensuring Reliability and Confidence
We help you maintain confidence in your oligonucleotide-based products by providing professional and standardized quality control solutions. Our services are designed to verify product integrity, identify potential issues, and ensure optimal performance in downstream applications. This service is not for diagnostic purposes, but rather to demonstrate that your primers, probes, and controls work as intended and to pinpoint sources of experimental issues.
Our Quality Control Methods
We offer multiple quality control methods to ensure the performance and purity of your requested oligonucleotides. This information helps you determine which QC method is most suitable for your specific products:
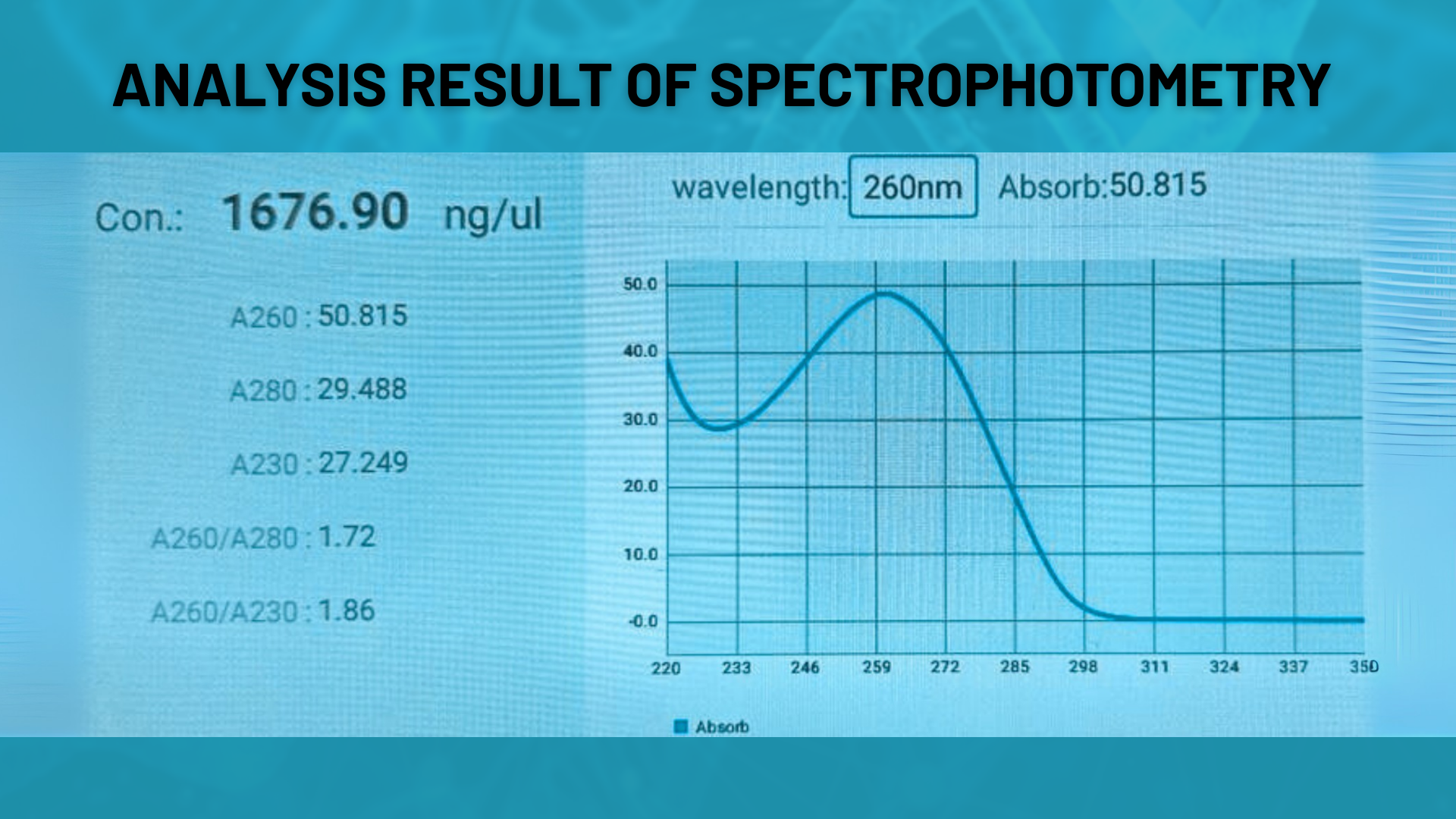
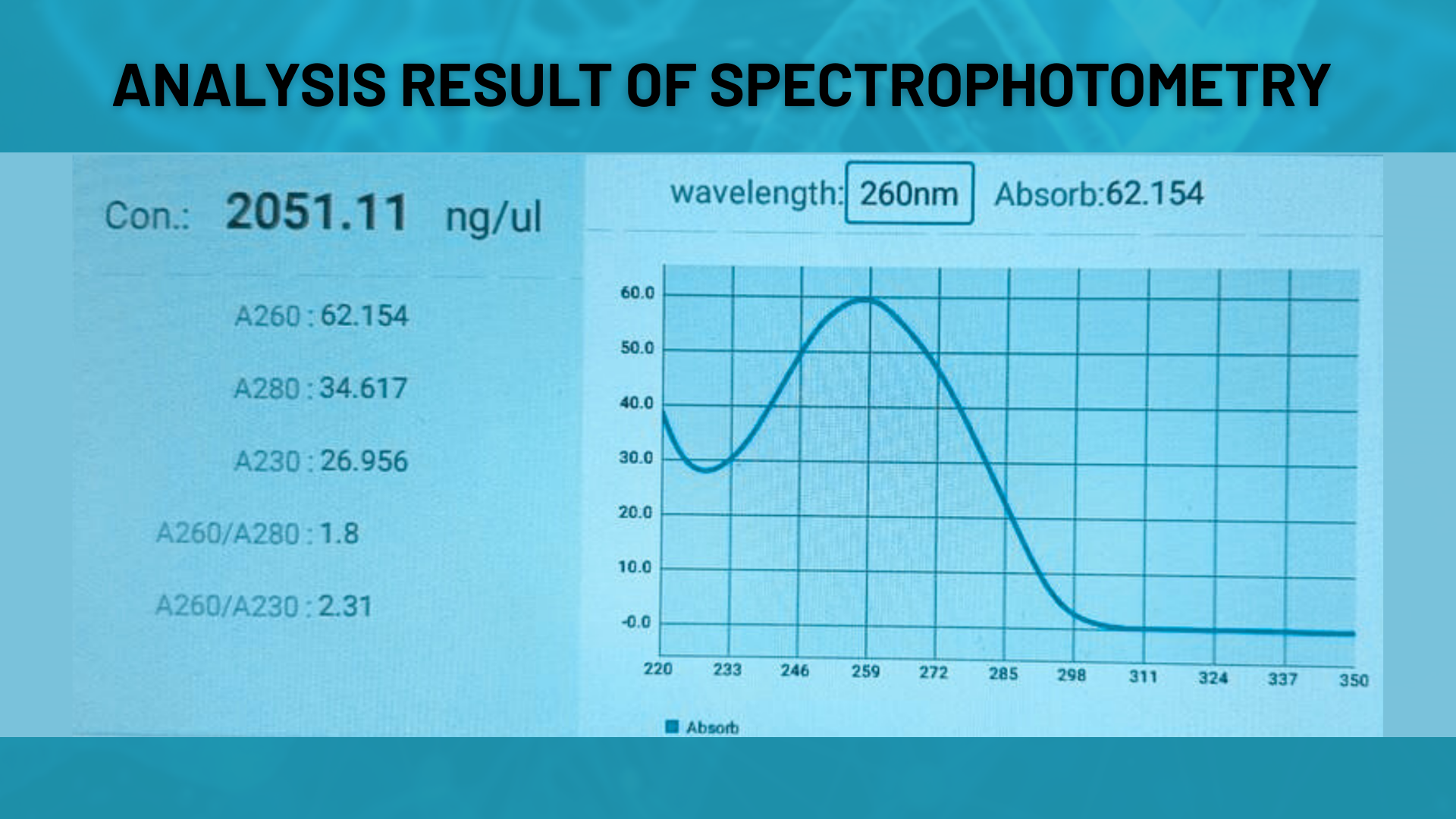
Spectrophotometric Analysis
Concentration and purity of oligonucleotides are assessed via UV absorbance measurements (A260, OD, nmol). This method provides a quick and reliable preliminary quality check.
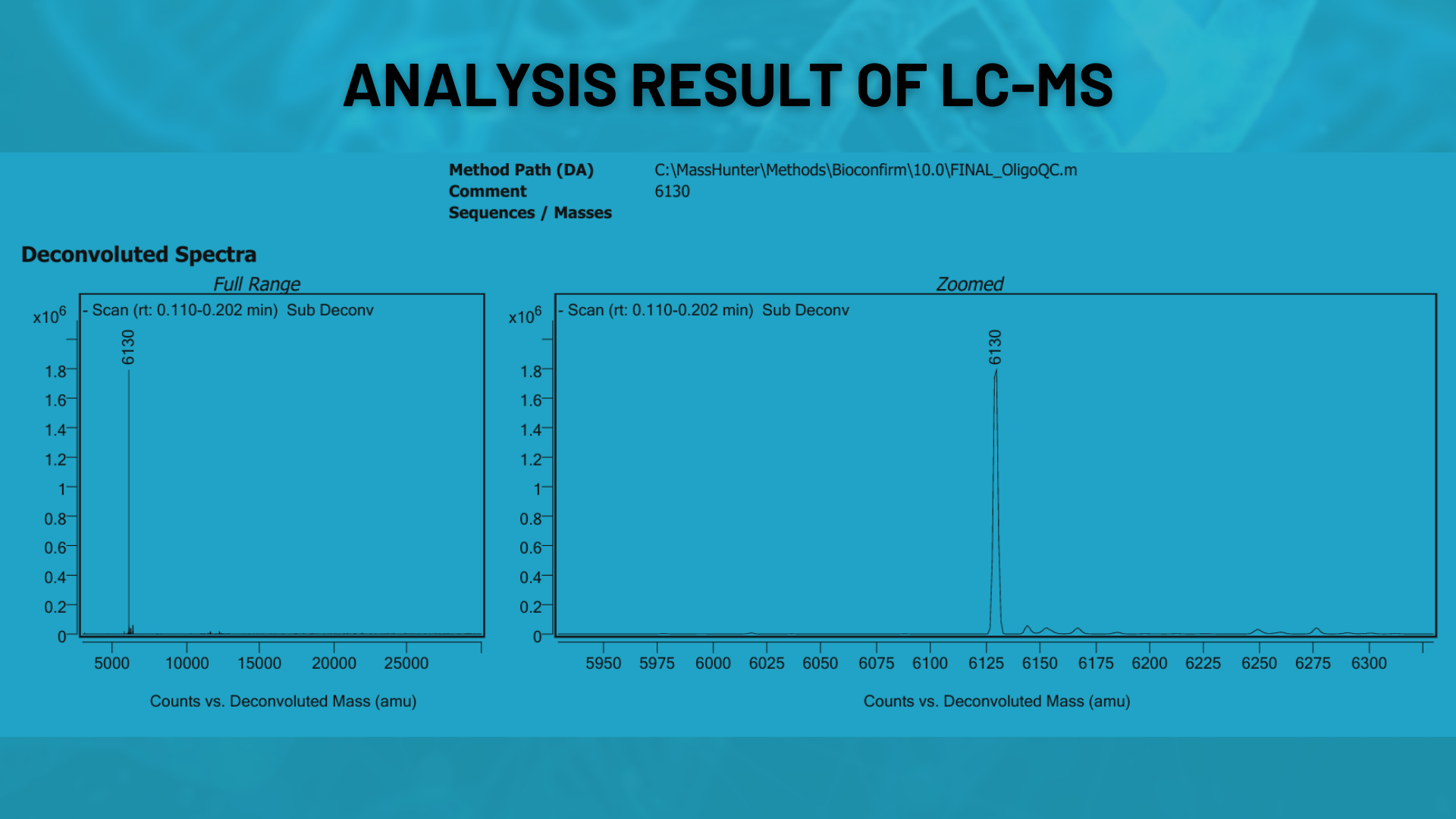
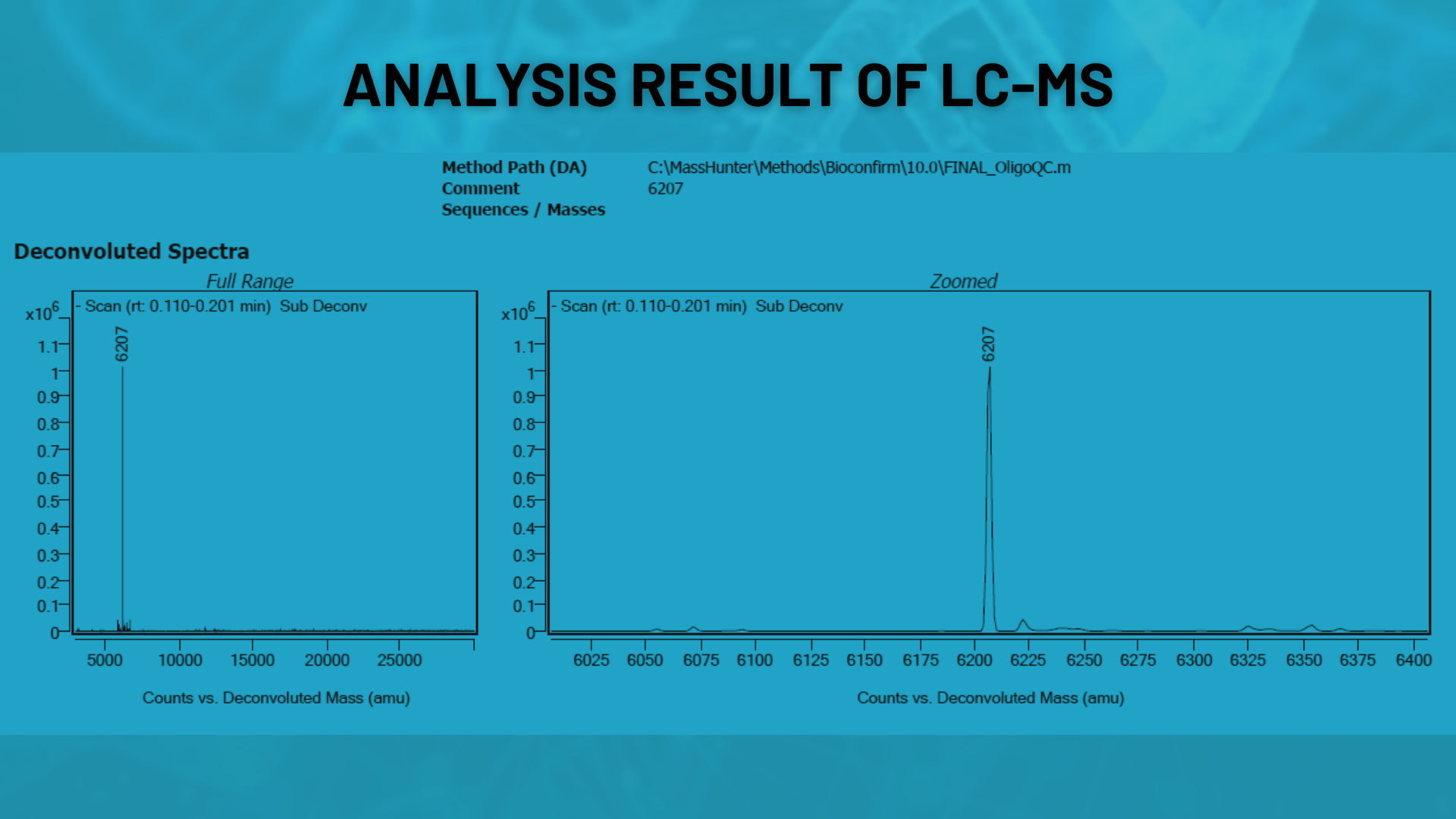
LC-MS (Mass Spectrometry)
The correct molecular mass of oligonucleotides is verified using LC-MS. This ensures the synthesized sequence is accurate and detects any incorrect products.
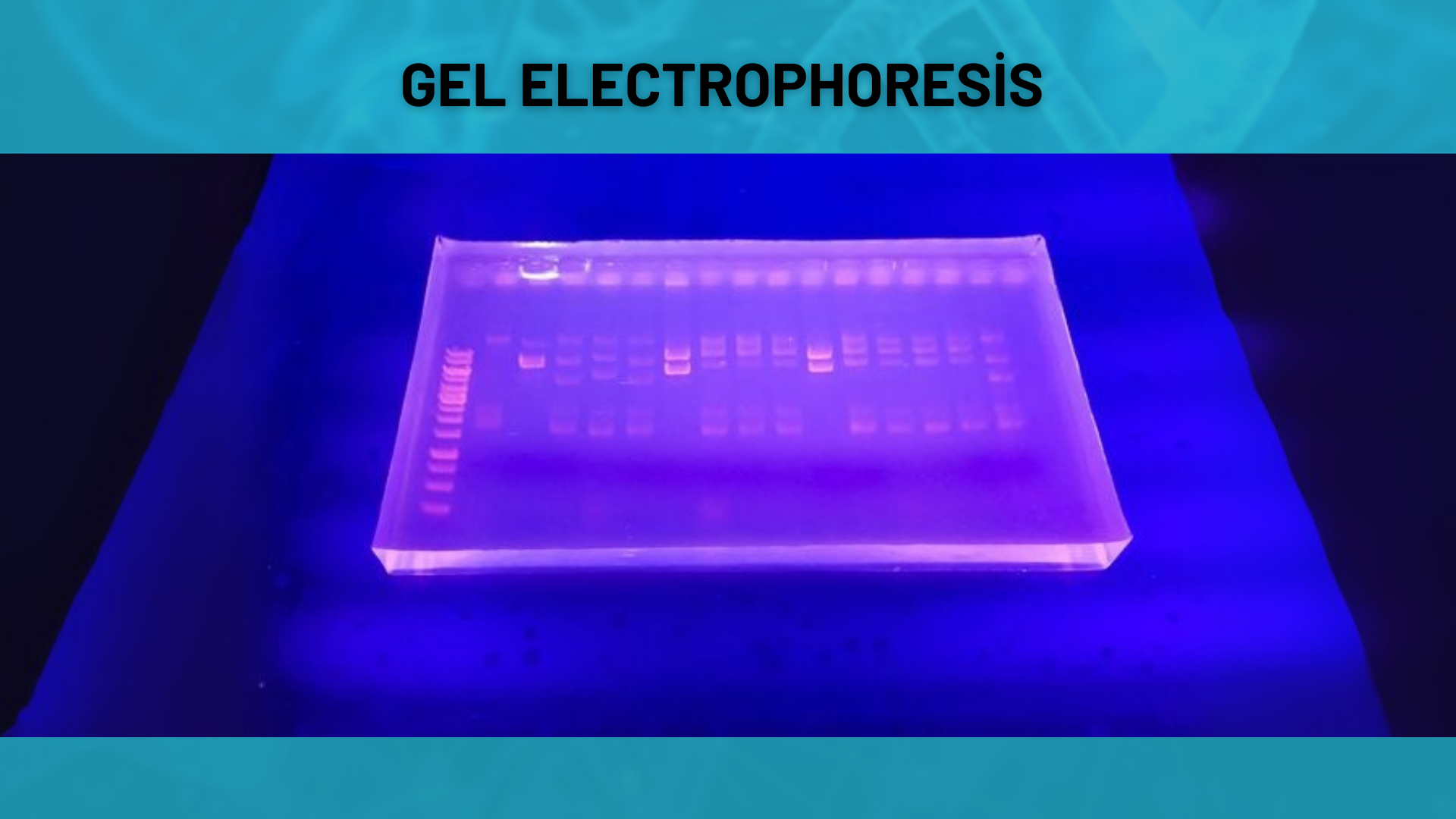
Gel Electrophoresis
The size and purity of oligonucleotides are visualized and analyzed on agarose or polyacrylamide gels. This method is mainly used when purity verification is required.
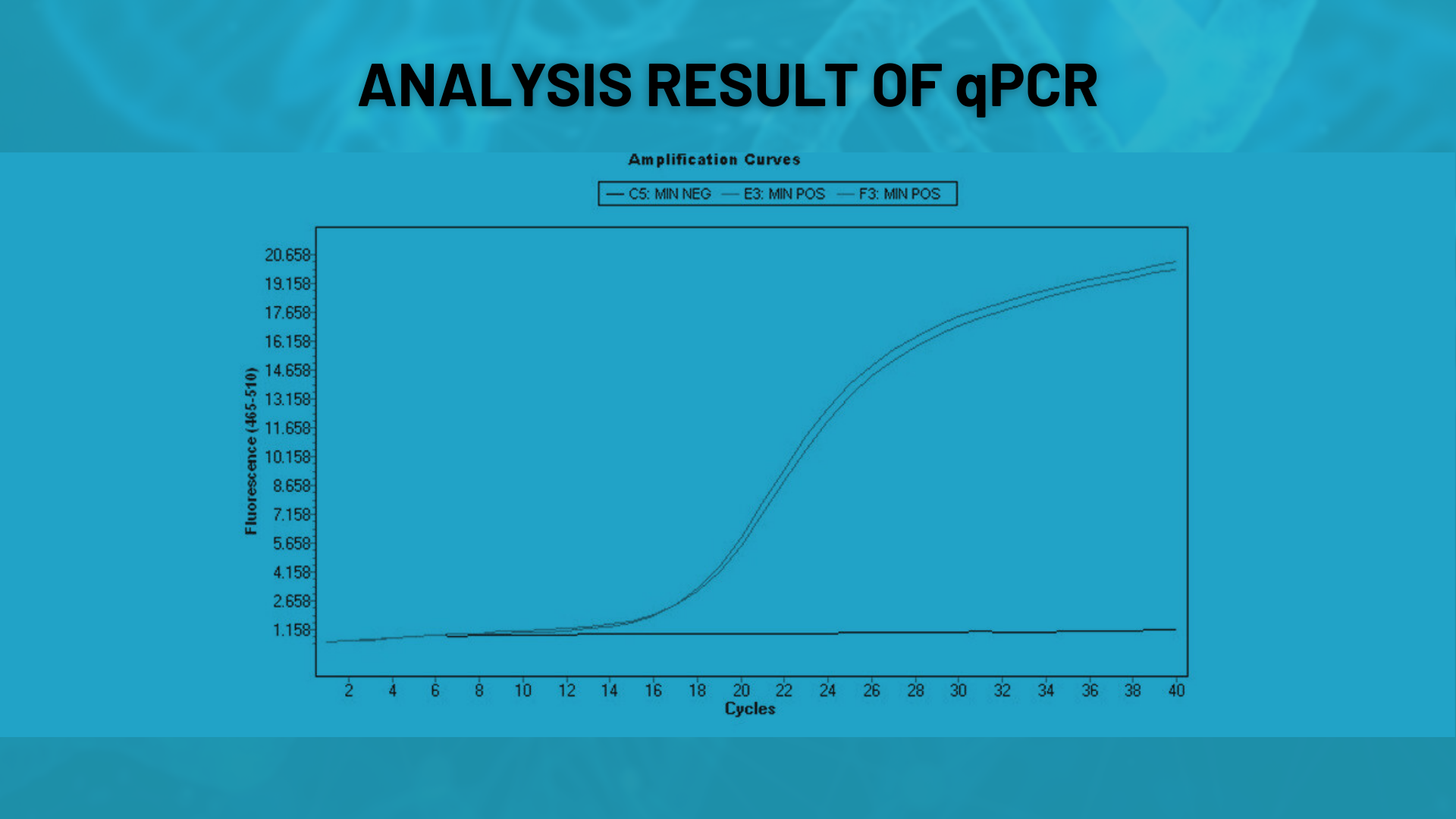
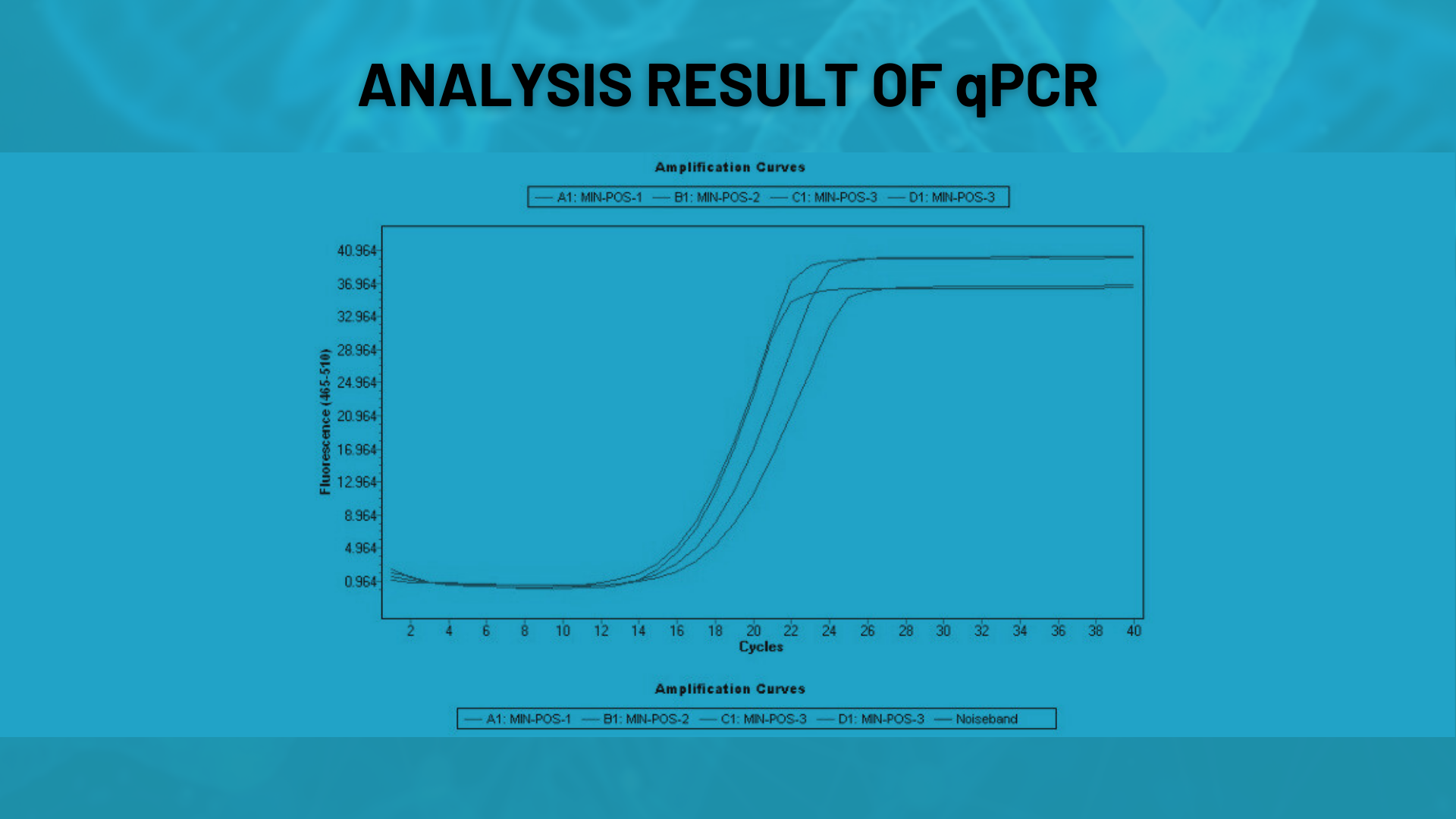
qPCR
Functional tests assess whether oligonucleotides perform correctly in PCR or qPCR applications. This method is particularly used to validate the efficiency of probe or primer sets.
Advantages of Choosing Us
- Experienced technical team ensures accurate and reliable QC
- Comprehensive reporting with actionable insights
- Rapid turnaround minimizes downtime in experimental workflows
- Support for troubleshooting and optimization of oligonucleotide performance
Quality Control Service Guide
1. Select Your QC Method
Identify the quality control method best suited for detecting or validating your target.
2. Provide the Necessary Materials
Ensure that all required oligonucleotides or nucleic acids (primer, probe, DNA, etc.) are supplied so the selected QC method can be applied effectively.
3. Specify Your Purpose
In the request form, provide detailed information about why the QC method is being used to ensure results are tailored to your experimental goals.
4. Receive Detailed Reports
After QC completion, you will receive a comprehensive report including all relevant data, such as mass, purity, integrity, and functional validation if applicable.